Use of Single Dose L-Isoleucine and L-Valine as part of the emergency management for Maple Syrup Urine Disease (MSUD) | Case Study
Description
A case study demonstrating the use of both Isoleucine1000 and Valine1000 in the emergency management of an infant with MSUD.
The patient is an 8 day old male diagnosed with Maple Syrup Urine Disease (MSUD).
Medical History
Initial presentation:
Presented to Emergency Department on request of Metabolic team after newborn screening result indicated likely diagnosis of MSUD.
Relevant History
Biochemistry:
Plasma L-leucine rose from 1050μmol/L (day 2 of life) to 2200μmol/L (on admission).
Clinical/Medications:
Hypertonic, back arching, and irritable. Non-encephalopathic. Seizures on day of admission - prescribed phenobarbital.
Dietary:
Formula-fed from birth. Parents reported progressively reduced oral intake over the 2-3 days before admission. Taking very small amounts frequently.
Overall Aim/Goal
Provide adequate energy to prevent catabolism and promote weight gain. Provide branched chain amino acid (BCAA) free amino acid-based formula for infants. Educate family on dietary management of MSUD.
Nutritional Requirements
Estimated energy requirement: 120kcal/kg/d.
Estimated total protein requirement: 2-3g/kg/d. Provide as BCAA-free amino acids +/- natural protein, as clinically appropriate.
Dietetic Intervention
Commenced standardised MSUD emergency regimen as per local protocol.
BCAA-free formula for infants + Valine1000 (Vitaflo) + Isoleucine1000 (Vitaflo).
Valine1000 and Isoleucine1000 made with water into separate 1% solutions (10mg of amino acid per ml). Both solutions added to 24-hour feed volume* to provide 50mg/kg/d of L-valine and 30mg/kg/d of L-isoleucine. Feeds given initially via nasogastric tube (NGT).
Ongoing Management
Ongoing monitoring and adjustment of feeding plan based on plasma BCAA levels and growth. Once appropriately well and alert, oral feeding was resumed, with top-ups via NGT. NGT top-ups used until plasma L-leucine was within the desired range, after which time the infant was able to take all feeds orally.
Monitoring and Review
Infant received 90-100% of estimated energy requirement each day of admission, as calculated via dietitian review. No feed intolerance was noted. Natural protein was reintroduced 42 hours after admission and increased by 0.5g/d until plasma L-leucine stabilised in the desired range of 100-200μmol/L (approx. 4g/d natural protein provided by standard infant formula). Infant was receiving all nutrition orally on discharge.
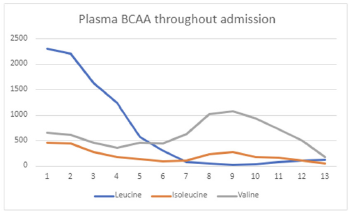
Outcome Measures
Plasma BCAA levels improved.
Main Findings/Product use
Use of Valine1000 and Isoleucine1000 sachets helps with accurate dosing for infants/ small body weight. Products dissolve well in water.
The standard 10mg/ml solutions of L-valine and L-isoleucine from the Valine1000/Isoleucine1000 sachets can be frozen and then thawed*, to add to the feeds of patients admitted after hours. This facilitates the rapid intervention required in such cases and reduces the potential risk of error involved in weighing powders at ward level.
Strengths, Limitations and Barriers
Time delay in processing of blood samples can pose challenges in a clinical setting. At times, dietary changes are made based on anticipated results to ensure that adjustments to the management plan are initiated and reviewed promptly.
Providing supplementation of L-valine and L-isoleucine in emergency management of MSUD is an essential part of ensuring optimal management of L-leucine levels and maintaining adequate plasma levels of L-valine and L-isoleucine for growth and development in the longer term, where adequate amounts may not be obtained from natural protein in the diet.
Pre-measured sachets (in this case containing 1000mg of amino acid per sachet) are easily made into a standardised solution (1 sachet made up to 100ml with water provides 10g of amino acid per ml). This, in turn, allows for accurate titration of dose and easier administration. Freezing these standardised solutions is useful for subsequent after-hours admission*.
Southeast Newborn Screening and Genetics Collaborative (SERC) and Genetic and Metabolic Dietitians International (GMDI) (2017). The Nutrition Management Guideline for Individuals with Maple Syrup Urine Disease (MSUD) Retrieved 21 March 2022 from: https://managementguidelines.net/guidelines.php/129/overview/0/0/MSUD%20Nutrition%20Guidelines/Version%201.58/Overview#Content%20Highlights
National Health and Medical Research Council (NHMRC) (2015). Nutrient reference values for Australia and New Zealand. Canberra: NHMRC. Retrieved 21 March 2022 from https://www.nrv.gov.au/.
* The Central Formula Room (RCH) understands the need for quality hygiene and safety provided for their clients and ensures that the food legislative requirements as described in the Victorian Food Act 1984 and Food Safety Standards (Chapter 3 of the Food Standard Code) are maintained. This includes (but is not limited to) correct temperature storage where potentially hazardous formula/EBM/oral supplements are stored in safe conditions at specified temperatures to protect food safety, and storage temperatures are monitored and documented.


