Basecal® | Case Study
DESCRIPTION
A case study on the dietary management of a 10-year old boy with Arginosuccinic Aciduria (ASA) using basecal.
Anthropometry:
- Weight: 33.4kg, 50th-75th centile
- Height: 123.7cm, 0.4th -2nd centile
Initial presentation:
Our case is the second child of unrelated Caucasian-origin parents. At 5 days of age he was admitted at a local hospital with respiratory symptoms and presumed sepsis. On follow-up he had neurological investigations for developmental delay.
Following sedation for an MRI scan, he became encephalopathic with ammonia elevated at 350µmol/L. He was started on 10% IV dextrose and ammonia concentrations reduced to 70µmol/L. Further analysis of urine and plasma showed an elevated level of argininosuccinic acid (urine) and low arginine (plasma) - the patient was diagnosed with ASA at 4 months of age.
A low protein diet and oral medications (sodium benzoate and arginine) commenced at diagnosis. He was then transferred to an inherited metabolic disorders (IMD) team for ongoing care.
Medications:
ASA, a urea cycle disorder (UCD), is a complex condition requiring a multi-faceted dietary and medicinal management regimen. In addition to the IMD team, the patient is reviewed by several other medical disciplines (e.g. liver). His prescription medications include the “nitrogen scavenging” medications sodium benzoate and sodium phenylbutyrate. He is also on:
- L-Arginine: 750mg TDS
- Omeprazole: 20mg OD
- Levetiracetam: 750mg BD
- Clonazepam: 600mcg OD
- Gaviscon liquid: 5mls after feeds
- Potassium Chloride: 10mmols BD
His dietary management involves following a low protein diet to meet WHO/FAO/UNU 2007 safe levels of protein intake1 which is achieved through oral intake and a low protein gastrostomy feed given day and night. Adequate energy intake is essential to achieve good metabolic control. In times when the oral intake is minimal, all his nutritional intake comes from enteral feeds. Emergency regimens, when required, are glucose polymer feeds (S.O.S20TM, Vitaflo).
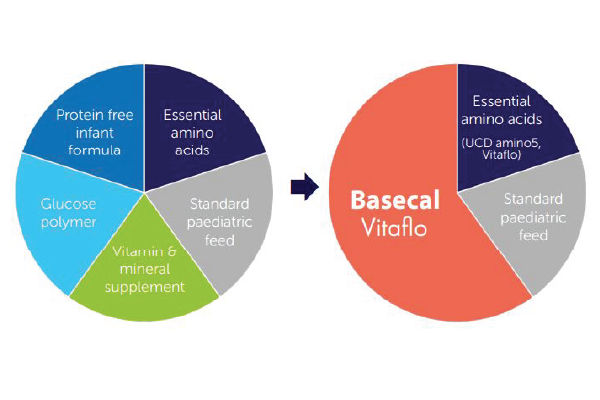
Prior to the introduction of basecal®, the patient was on a complex modular feed, comprised of multiple ingredients which required regular adjustments.
With the introduction of basecal, a protein free feed containing carbohydrate, fat and micronutrients, there was an opportunity to simplify this aspect of his management.

Although the new and old feed recipes were isocaloric, the introduction of basecal simplified the patient’s feeding regimen. The patient tolerated the new tube feed well - there was no feed intolerance. His ammonia levels were stable and his blood essential amino acids levels were mainly within reference range.
The feeding plan has been followed well and has proved easily adaptable to changes in the patient’s appetite, growth needs, nutritional requirements and metabolic stability.
The patient’s main carer is a single, working mother and this increased ease of preparation has proved very valuable. Although the patient’s behaviour remains challenging, periods of metabolic instability and inpatient admissions became less frequent.
Furthermore, his appetite has improved, reducing his overall dependence on tube-feeding and eliminating the need for any supplementary daytime gastrostomy feeds which previously had sometimes been necessary.
This case study highlights several familiar challenges for those involved in the dietary management of UCD;
- Difficulties and delays in diagnosis
- A significant and chronic disease burden
- Complexity of dietetic and medical management
With regard to this last point, dietitians can play a pivotal role in reducing dietary management complexity. In patients requiring bespoke modular feeds, research has indicated that a regimen with fewer ingredients is more accurate and easier for caregivers to prepare2,3,4.
In this case, basecal helped reduce the number of ingredients in the patient’s feed. It was well accepted by the patient orally, so it boosted oral intake which, in combination with his improvements in appetite, helped reduce reliance on enteral feeds. With metabolic patients at risk of metabolic decompensation, it is noteworthy that these practical benefits were accompanied by good gastro-intestinal tolerance and improved metabolic stability.
- Häberle J, Boddaert N, Burlina, A, Chakrapani A, Dixon M, Huemer M et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2017; 7(1):32.
- Daly A, Evans S, Ashmore C, Chahal S, Santra S, MacDonald, A. The challenge of nutritional profiling of a protein-free feed module for children on low protein tube feeds with organic acidaemias. J Hum Nutr Diet. 2017; 30(3): 292-301.
- Daly A, Evans S, Ashmore C, Chahal S, Santra S, MacDonald A. Refining low protein modular feeds for children on low protein tube feeds with organic acidaemias. Mol Genet Metab Rep. 2017; 13: 99-104.
- Evans S, Daly A, Ashmore C, Gokmen-Ozel H, Dileva R, Dumbleton B et al. Nutritional content of modular feeds: how accurate is feed production? Arch Dis Child. 2013; 98(3):184-8.
